
Welcome to Anavex Life Sciences Corp. Our SIGMACEPTOR™ Discovery Platform produces therapeutic candidates designed to activate SIGMAR1, a novel pathway designed to target chronic central nervous system (CNS) conditions with genomic precision. At Anavex Life Sciences, we are dedicated to improving overall quality of life for people living with neurodegenerative and neurodevelopmental disorders. Our product portfolio platform is currently dedicated to solving the following list of life-altering conditions.
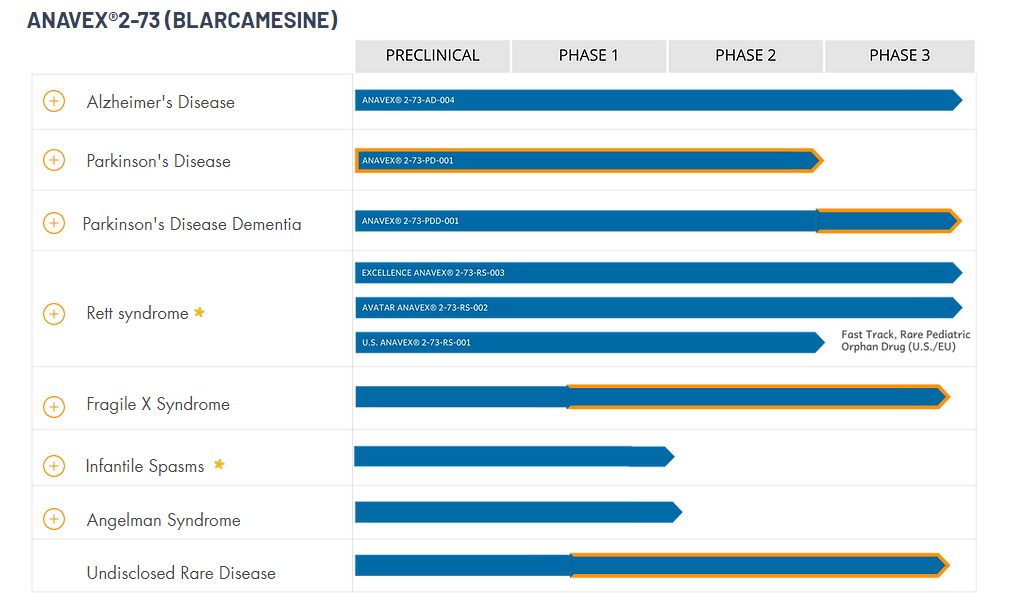
Anavex Life Sciences Corp., a clinical stage biopharmaceutical company, engages in the development of drug candidates for the treatment of central nervous system diseases. The company’s lead drug candidate is ANAVEX 2-73, which is in Phase III clinical trial for the treatment of Alzheimer’s disease; Phase II clinical trials to treat Parkinson’s disease; and preclinical clinical trials to treat Rett syndrome, epilepsy, infantile spasms, Fragile X syndrome, Angelman syndrome, multiple sclerosis, and tuberous sclerosis complex. Its preclinical drug candidates include ANAVEX 3-71, a central nervous system (CNS)-penetrable mono-therapy to treat Alzheimer’s and Parkinson’s diseases; ANAVEX 1-41, a sigma-1 agonist for the treatment of depression, stroke, Parkinson’s, and Alzheimer’s diseases; ANAVEX 1066, a mixed sigma-1/sigma-2 ligand for the potential treatment of neuropathic and visceral pain; and ANAVEX 1037 to treat prostate and pancreatic cancer. The company is based in New York, New York.
Anavex utilizes precision genetic medicine to treat severe and devastating neurological disorders and is focusing on rare diseases with no available therapy (Rett syndrome) as well as neurodegenerative diseases that are on the rise due to aging populations (Parkinson’s Disease and Alzheimer’s Disease)
Proof of Concept Controlled Phase 2 Clinical Trial Data Evaluating ANAVEX®2-73 (blarcamesine) in Parkinson’s Disease Dementia Presented at CTAD 2020 Conference
Statistically significant improvements in CDR system Cognitive Domain of Attention assessed by Choice Reaction Time (p = 0.039) and Digital Vigilance (p = 0.008)
Statistically significant dose-dependent improvements in Episodic Memory (p = 0.003)
ANAVEX®2-73 (blarcamesine) prevented the on-going cognitive decline in treated patients compared to placebo
Late breaking abstract of cognitive outcome measures relevant to Alzheimer’s disease selected for oral presentation at CTAD
NEW YORK – November 6, 2020 – Anavex Life Sciences Corp. (“Anavex” or the “Company”) (Nasdaq: AVXL), a clinical-stage biopharmaceutical company developing differentiated therapeutics for the treatment of neurodegenerative and neurodevelopmental disorders including Alzheimer’s disease (AD), Parkinson’s disease (PD), Rett syndrome and other central nervous system (CNS) diseases, today announced additional details on and presented the results from the proof of concept Phase 2 controlled trial evaluating the safety, tolerability, and efficacy of ANAVEX®2-73 (blarcamesine) in patients with Parkinson’s disease dementia (PDD) at the 13th international conference on Clinical Trials on Alzheimer’s Disease (CTAD). CTAD is an annual conference focused on Alzheimer’s research and development and takes place this year as a virtual event on November 4-7th, 2020.
Details of the Late-breaking Presentation:
Title: “ANAVEX®2-73 (blarcamesine) Currently in Phase 2b/3 Early Alzheimer’s Disease (AD): Analysis of Cognitive Outcome Measures Relevant to AD of Double-blind, Multicenter, Placebo-controlled Phase 2 Clinical Trial in 132 Patients with Parkinson’s Disease Dementia”
| Presentation Type: | Late-Breaking, Oral Presentation (LB25) |
| Presenter: | Dag Aarsland, MD, PhD – King’s College London, UK |
| Date/Time: | November 6, 2020, 10:45 am EST |
The study found that ANAVEX®2-73 (blarcamesine) was well tolerated in oral doses up to 50 mg once daily. The results showed clinically meaningful, dose-dependent, and statistically significant improvements in the Cognitive Drug Research (CDR) computerized assessment system analysis. The study validated the precision medicine approach of targeting SIGMAR1 as a genetic biomarker of response to ANAVEX®2-73 (blarcamesine), confirming that ANAVEX®2-73 (blarcamesine) acts through SIGMAR1 activation. These results support continued development in PDD / PD as well as the currently ongoing Phase 2 and Phase 2/3 clinical studies with ANAVEX®2-73 (blarcamesine) in Rett syndrome[1] and Alzheimer’s disease[2].
Highlights of the study results:
- Broad and statistically significant improvements in CDR system Cognitive Domain of Attention assessed by Choice Reaction Time (p = 0.039) and Digital Vigilance (p = 0.008) and CDR system Episodic Memory (p = 0.047), representing complex cognitive tasks with impact on quality of life such as making a choice between similar objects and remembering daily personal experiences, which are mostly impaired in both PD and AD.[3]
- Statistically significant dose-dependent (p = 0.003) improvement of Episodic Memory, which has been shown to be highly correlated (70%) with the Alzheimer’s Disease Assessment Scale–Cognitive score (ADAS-Cog; r = 0.7).[4]
- ANAVEX®2-73 (blarcamesine) does not impair sleep and has a positive effect on REM sleep behavior disorder.
- ANAVEX®2-73 (blarcamesine) was generally safe, well tolerated, and improved safety profile compared to dementia drugs associated with typical adverse effects.
The presentation is available on the Anavex website (www.anavex.com).
The ANAVEX®2-73-PDD-001 study was an international, double-blind, multicenter, placebo-controlled proof of concept Phase 2 clinical study that randomized 132 patients with PDD equally to target doses of 30mg, 50mg ANAVEX®2-73 (blarcamesine) or placebo, respectively. In addition to safety and cognitive efficacy, sleep function was assessed during the study at week 8 and week 14.
ANAVEX®2-73-PDD-001 study results will be submitted for publication in a peer-reviewed medical journal. Anavex is planning a pivotal trial of ANAVEX®2-73 (blarcamesine) in Parkinson’s disease dementia after submitting the results of the study to the FDA to obtain regulatory guidance.
“I am very intrigued to see the promising results of the ANAVEX®2-73-PDD-001 trial, providing significant improvements in cognitive function accompanied by a favorable safety and tolerability profile,” said Dag Aarsland, MD, PhD, Professor and the Head of Department of Old Age Psychiatry at the Institute of Psychiatry, Psychology & Neuroscience, King’s College London, UK. “The ANAVEX®2-73 (blarcamesine) study results represent a meaningful step forward toward urgently needed treatment for this serious complication of Parkinson’s disease given that cognitive impairment of patients with Parkinson’s disease dementia is very distressing to patients and their families and is associated with greater risk of institutionalization and accelerated progression to severe dementia and death.”
Dr. Jaime Kulisevsky, MD, PhD, Full Professor of Neurology & Vice-Dean Faculty of Medicine Autonomous University of Barcelona and Director of the Movement Disorders Unit, Department of Neurology, Sant Pau Hospital and Principal Investigator in the trial, commented, “PDD is a debilitating disorder with significant co-morbidities and there has not been a mechanistically novel medication approved for PDD in over 20 years. Hence, new therapies are urgently needed to alleviate this suffering and disability. As the first double-blind trial of ANAVEX®2-73 (blarcamesine) in PDD, this proof-of-concept study provides very encouraging and clinically relevant data.”
Christopher U Missling, PhD, President & Chief Executive Officer of Anavex, “Our strategy has been consistently to advance ANAVEX®2-73 (blarcamesine) with focus on Precision Medicine and to validate this approach in clinical studies in patients with significant cognitive impairments. We are pleased with these PDD study results that will be further supplemented by actigraphy movement data and whole genome exome DNA and RNA data. Finally, we would like to thank all the patients and participating families as well the investigators and clinical site coordinators for their dedication to this study.”
After completing the trial, participants were able to enroll in a voluntary 48-week open-label extension study, ANAVEX®2-73-PDD-EP-001, which continues to assess safety, long term efficacy and changes in gut microbiota.[5]
ANAVEX®2-73 (blarcamesine) is an orally available, small-molecule activator of the sigma-1 receptor (SIGMAR1), which has been shown to be pivotal to restoring neural cell homeostasis and promoting neuroplasticity.[6]
About Parkinson’s Disease Dementia (PDD)
Parkinson’s disease is a fairly common neurological disorder in older adults, estimated to affect nearly 2 percent of those older than age 65. The Parkinson’s Foundation estimates that 1 million Americans have Parkinson’s disease. It is estimated that up to 80 percent of those with Parkinson’s disease eventually experience Parkinson’s disease dementia. The brain changes caused by Parkinson’s disease begin in a region that plays a key role in movement. As Parkinson’s brain changes gradually spread, they often begin to affect mental functions, including memory and the ability to pay attention, make sound judgments and plan the steps needed to complete a task.[7]
About ANAVEX®2-73 (blarcamesine)
ANAVEX®2-73 (blarcamesine) activates the Sigma-1 receptor (S1R) protein, which serves as a molecular chaperone and functional modulator involved in restoring homeostasis. In a Phase 2a Alzheimer’s disease (AD) study, ANAVEX®2-73 (blarcamesine) has shown dose dependent improvement in exploratory endpoints of cognition (MMSE) and activities of daily living (ADCS-ADL). Full genomic analysis of ANAVEX®2-73 (blarcamesine) Phase 2a study in AD patients was performed. The ANAVEX®2-73 (blarcamesine) Phase 2 PDD study design includes genomic biomarkers identified in the ANAVEX®2-73 (blarcamesine) Phase 2a AD study. Studies of ANAVEX®2-73 (blarcamesine) in a disease modifying animal model of Parkinson’s disease indicates that ANAVEX®2-73 (blarcamesine) is well tolerated, induces significant motor recovery (p<0.05), induces neurohistological restoration (p<0.05) and reduces microglial activation (p<0.05), a potential biomarker of Parkinson’s disease. Behavioral patterns were completely normal, meaning no signs of either dystonia or stereotypic behaviors were detected in animals receiving the treatment. These studies were funded by The Michael J. Fox Foundation for Parkinson’s Research.
About Anavex Life Sciences Corp.
Anavex Life Sciences Corp. (Nasdaq: AVXL) is a publicly traded biopharmaceutical company dedicated to the development of differentiated therapeutics for the treatment of neurodegenerative and neurodevelopmental disorders including Alzheimer’s disease, Parkinson’s disease, Rett syndrome and other central nervous system (CNS) diseases, pain and various types of cancer. Anavex’s lead drug candidate, ANAVEX®2-73 (blarcamesine), recently completed a successful Phase 2a clinical trial for Alzheimer’s disease. ANAVEX®2-73 (blarcamesine) is an orally available drug candidate that restores cellular homeostasis by targeting sigma-1 and muscarinic receptors. Preclinical studies demonstrated its potential to halt and/or reverse the course of Alzheimer’s disease. ANAVEX®2-73 (blarcamesine) also exhibited anticonvulsant, anti-amnesic, neuroprotective and anti-depressant properties in animal models, indicating its potential to treat additional CNS disorders, including epilepsy. The Michael J. Fox Foundation for Parkinson’s Research previously awarded Anavex a research grant, which fully funded a preclinical study to develop ANAVEX®2-73 (blarcamesine) for the treatment of Parkinson’s disease. ANAVEX®3-71, which targets sigma-1 and muscarinic receptors, is a promising preclinical drug candidate demonstrating disease-modifying activity against the major hallmarks of Alzheimer’s disease in transgenic (3xTg-AD) mice, including cognitive deficits, amyloid and tau pathologies. In preclinical trials, ANAVEX®3-71 has shown beneficial effects on mitochondrial dysfunction and neuroinflammation. Further information is available at www.anavex.com. You can also connect with the company on Twitter, Facebook and LinkedIn.
Forward-Looking Statements
Statements in this press release that are not strictly historical in nature are forward-looking statements. These statements are only predictions based on current information and expectations and involve a number of risks and uncertainties. Actual events or results may differ materially from those projected in any of such statements due to various factors, including the risks set forth in the Company’s most recent Annual Report on Form 10-K filed with the SEC. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement and Anavex Life Sciences Corp. undertakes no obligation to revise or update this press release to reflect events or circumstances after the date hereof.
For Further Information:
Anavex Life Sciences Corp.
Research & Business Development
Toll-free: 1-844-689-3939
Email: info@anavex.com
Investors & Media:
Email: ir@anavex.com
[1] ClinicalTrials.gov Identifiers: NCT03758924, NCT03941444, NCT04304482
[2] ClinicalTrials.gov Identifiers: NCT03790709, NCT02756858
[3] Mahurin, R. K., & Pirozzolo, F. J. (1993). Application of Hick’s law of response speed in Alzheimer and Parkinson diseases. Perceptual and Motor Skills, 77(1), 107–113
[4] Wesnes K, Edgar C, Andreasen N, Annas P, Basun H, Lannfelt L, et al. Computerized cognition assessment during acetylcholinesterase inhibitor treatment in Alzheimer’s disease. Acta Neurol Scand 2010; 122:270–7
[5] ClinicalTrials.gov Identifier: NCT04575259
[6] Advances in Experimental Medicine and Biology Volume 964 (2017) Sigma Receptors: Their Role in Disease and as Therapeutic Targets.
[7] Source: https://www.alz.org/alzheimers-dementia/what-is-dementia/types-of-dementia/parkinson-s-disease-dementia
Anavex Life Sciences Announces Positive Results from Proof of Concept controlled Phase 2 Clinical Trial Evaluating ANAVEX®2-73 (blarcamesine) in Parkinson’s Disease Dementia
Clinically meaningful, dose-dependent, and statistically significant improvements in the Cognitive Drug Research (CDR) computerized assessment system analysis
Company to host conference call today at 8:30 AM ET
NEW YORK – October 15, 2020 – Anavex Life Sciences Corp. (“Anavex” or the “Company”) (Nasdaq: AVXL), a clinical-stage biopharmaceutical company developing differentiated therapeutics for the treatment of neurodegenerative and neurodevelopmental disorders including Alzheimer’s disease (AD), Parkinson’s disease (PD), Rett syndrome and other central nervous system (CNS) diseases, today announced results from the proof of concept Phase 2 controlled trial evaluating the safety, tolerability, and efficacy of ANAVEX®2-73 (blarcamesine) in patients with Parkinson’s disease dementia (PDD).
The study found that ANAVEX®2-73 (blarcamesine) was safe and well tolerated in oral doses up to 50 mg once daily. The results show clinically meaningful, dose-dependent, and statistically significant improvements in the Cognitive Drug Research (CDR) computerized assessment system analysis. The study confirmed the precision medicine approach of targeting SIGMAR1 as a genetic biomarker of response to ANAVEX®2-73 (blarcamesine).
The ANAVEX®2-73-PDD-001 study was an international, double-blind, multicenter, placebo-controlled Phase 2 clinical study and randomized 132 patients with PDD equally to target doses of 30mg, 50mg ANAVEX®2-73 (blarcamesine) or placebo, respectively. In addition to safety and cognitive efficacy, sleep function was assessed during the study at week 8 and week 14.
ANAVEX®2-73-PDD-001 study results will be submitted for presentation at a medical conference and for publication in a peer-reviewed medical journal. Anavex is planning a pivotal trial of ANAVEX®2-73 (blarcamesine) in Parkinson’s disease dementia after submitting the results of the study to the FDA to obtain regulatory guidance.
Dr. Jaime Kulisevsky Bojarski, MD, PhD, Full Professor of Neurology & Vice-Dean Faculty of Medicine Autonomous University of Barcelona and Director of the Movement Disorders Unit, Department of Neurology, Sant Pau Hospital and Principal Investigator in the trial, commented, “As the first double-blind trial of ANAVEX®2-73 (blarcamesine) in PDD, this proof-of-concept study provides extremely encouraging data. PDD can be debilitating with significant co-morbidities. New therapies are urgently needed to alleviate this suffering and disability. There has not been a mechanistically novel medication approved for PDD in over 20 years.”
“I am very pleased to see the promising results of the ANAVEX®2-73-PDD-001 trial, providing significant improvements in cognitive function accompanied by a favorable safety and tolerability profile,” said Dag Aarsland, MD, PhD, Professor and the Head of Department of Old Age Psychiatry at the Institute of Psychiatry, Psychology & Neuroscience, King’s College London, UK. “Cognitive impairment of patients with Parkinson’s disease dementia is very distressing to patients and their families and is associated with greater risk of institutionalization and accelerated progression to severe dementia and death. Given the limited options of adequate treatments for Parkinson’s disease dementia, and the safety concerns and modest or uncertain efficacy of currently used off-label treatments, the ANAVEX®2-73 (blarcamesine) study results represent a meaningful step forward toward urgently needed treatment for this serious complication of Parkinson’s disease.”
“We would like to thank all the patients and participating families as well the investigators and clinical site coordinators for their dedication to this study”, said Christopher U. Missling, PhD, President and Chief Executive Officer of Anavex. “Our strategy to advance ANAVEX®2-73 (blarcamesine) with focus on Precision Medicine has been validated in this study of patients with significant cognitive impairment and we are looking forward to the next clinical data readout of ANAVEX®2-73 (blarcamesine) in Rett syndrome and Alzheimer’s disease, indications where cognitive impairment is also prevalent.”
After completing the trial, participants were able to enroll in a voluntary 48-week open-label extension study, ANAVEX®2-73-PDD-EP-001, which continues to assess safety, long term efficacy and changes in gut microbiota.[1]
ANAVEX®2-73 (blarcamesine) is an orally available, small-molecule activator of the sigma-1 receptor, which has been shown to be pivotal to restoring neural cell homeostasis and promoting neuroplasticity.[2]
Conference Call Information
Anavex will host a conference call and webcast today at 8:30 AM Eastern Time to discuss the topline results of the ANAVEX®2-73-PDD-001 trial of ANAVEX®2-73 (blarcamesine) in Parkinson’s disease dementia. To participate in the live conference call, please dial 1 (866) 939-3921 (toll-free domestic) or 1 (678) 302-3550 (international) and use the passcode 49986272. The webcast can be accessed at https://wsw.com/webcast/cc/avxl15/1496358 and on the Company’s website at www.anavex.com. A replay of the webcast will be available for approximately 30 days following the live event.
About Parkinson’s Disease Dementia (PDD)
Parkinson’s disease is a fairly common neurological disorder in older adults, estimated to affect nearly 2 percent of those older than age 65. The Parkinson’s Foundation estimates that 1 million Americans have Parkinson’s disease. It is estimated that up to 80 percent of those with Parkinson’s disease eventually experience Parkinson’s disease dementia. The brain changes caused by Parkinson’s disease begin in a region that plays a key role in movement. As Parkinson’s brain changes gradually spread, they often begin to affect mental functions, including memory and the ability to pay attention, make sound judgments and plan the steps needed to complete a task.[3]
About ANAVEX®2-73 (blarcamesine)
ANAVEX®2-73 (blarcamesine) activates the Sigma-1 receptor (S1R) protein, which serves as a molecular chaperone and functional modulator involved in restoring homeostasis. In a Phase 2a Alzheimer’s disease (AD) study, ANAVEX®2-73 (blarcamesine) has shown dose dependent improvement in exploratory endpoints of cognition (MMSE) and activities of daily living (ADCS-ADL). Full genomic analysis of ANAVEX®2-73 (blarcamesine) Phase 2a AD patients was performed. The ANAVEX®2-73 (blarcamesine) Phase 2 PDD study design includes genomic biomarkers identified in the ANAVEX®2-73 (blarcamesine) Phase 2a AD study. Studies of ANAVEX®2-73 (blarcamesine) in a disease modifying model of Parkinson’s disease indicates that ANAVEX®2-73 (blarcamesine) is well tolerated, induces significant motor recovery (p<0.05), induces neurohistological restoration (p<0.05) and reduces microglial activation (p<0.05), a potential biomarker of Parkinson’s disease. Behavioral patterns were completely normal, meaning no signs of either dystonia or stereotypic behaviors were detected in animals receiving the treatment. These studies were funded by The Michael J. Fox Foundation for Parkinson’s Research.
About Anavex Life Sciences Corp.
Anavex Life Sciences Corp. (Nasdaq: AVXL) is a publicly traded biopharmaceutical company dedicated to the development of differentiated therapeutics for the treatment of neurodegenerative and neurodevelopmental disorders including Alzheimer’s disease, Parkinson’s disease, Rett syndrome and other central nervous system (CNS) diseases, pain and various types of cancer. Anavex’s lead drug candidate, ANAVEX®2-73 (blarcamesine), recently completed a successful Phase 2a clinical trial for Alzheimer’s disease. ANAVEX®2-73 (blarcamesine) is an orally available drug candidate that restores cellular homeostasis by targeting sigma-1 and muscarinic receptors. Preclinical studies demonstrated its potential to halt and/or reverse the course of Alzheimer’s disease. ANAVEX®2-73 (blarcamesine) also exhibited anticonvulsant, anti-amnesic, neuroprotective and anti-depressant properties in animal models, indicating its potential to treat additional CNS disorders, including epilepsy. The Michael J. Fox Foundation for Parkinson’s Research previously awarded Anavex a research grant, which fully funded a preclinical study to develop ANAVEX®2-73 (blarcamesine) for the treatment of Parkinson’s disease. ANAVEX®3-71, which targets sigma-1 and muscarinic receptors, is a promising preclinical drug candidate demonstrating disease-modifying activity against the major hallmarks of Alzheimer’s disease in transgenic (3xTg-AD) mice, including cognitive deficits, amyloid and tau pathologies. In preclinical trials, ANAVEX®3-71 has shown beneficial effects on mitochondrial dysfunction and neuroinflammation. Further information is available at www.anavex.com. You can also connect with the company on Twitter, Facebook and LinkedIn.

PRODUCT PIPELINE


AVXL DISCLAIMER
This newsletter is a paid advertisement, not a recommendation nor an offer to buy or sell securities. This newsletter is owned, operated and edited by MEC Systems LLC is a wholly owned subsidiary of milestonecgp.com, milestonecapitolgrowthportfolio.com
Any wording found in this e-mail, disclaimer or company profile referencing to “I” or “we” or “our” or “MEC Systems LLC” refers to MEC Systems LLC. Our business model is to be financially compensated to market and promote small public companies. By reading our newsletter and our website you agree to the terms of our disclaimer, which are subject to change at any time. We are not registered or licensed in any jurisdiction whatsoever to provide investing advice or anything of an advisory or consultancy nature, and are therefore are unqualified to give investment recommendations. Always do your own research and consult with a licensed investment professional before investing. This communication is never to be used as the basis of making investment decisions, and is for entertainment purposes only. At most, this communication should serve only as a starting point to do your own research and consult with a licensed professional regarding the companies profiled and discussed. Conduct your own research. Companies with low price per share are speculative and carry a high degree of risk, so only invest what you can afford to lose. By using our service you agree not to hold our site, its editor’s, owners, or staff liable for any damages, financial or otherwise, that may occur due to any action you may take based on the information contained within our newsletters or on our website.
We do not advise any reader take any specific action. Losses can be larger than expected if the company experiences any problems with liquidity or wide spreads. Our website and newsletter are for entertainment purposes only. Never invest purely based on our alerts. Gains mentioned in our newsletter and on our website may be based on end-of-day or intraday data. This publication and their owners and affiliates may hold positions in the securities mentioned in our profiles, which we may sell at any time without notice to our subscribers, which may have a negative impact on share prices. If we own any shares we will list the information relevant to the stock and number of shares here. We have been compensated $5,000k cash via bank wire by AVXL, to conduct Social Media Program and news distribution for AVXL from 9/18/2022 to 10/18/2022 . MEC Systems LLC business model is to receive financial compensation to advertise for public companies. This compensation is a major conflict of interest in our ability to be unbiased regarding. Therefore, this communication should be viewed as a commercial advertisement only. We have not investigated the background of the hiring third party or parties. The third party, profiled company, or their affiliates may wish to liquidate shares of the profiled company at or near the time you receive this communication, which has the potential to hurt share prices. Any non-compensated alerts are purely for the purpose of expanding our database for the benefit of our future financially compensated investor relations efforts. Frequently companies profiled in our alerts may experience a large increase in volume and share price during the course of investor relations marketing, which may end as soon as the investor relations marketing ceases. The investor relations marketing may be as brief as one day, after which a large decrease in volume and share price is likely to occur. Our emails may contain forward looking statements, which are not guaranteed to materialize due to a variety of factors.
We do not guarantee the timeliness, accuracy, or completeness of the information on our site or in our newsletters. The information in our email newsletters and on our website is believed to be accurate and correct, but has not been independently verified and is not guaranteed to be correct. The information is collected from public sources, such as the profiled company’s website and press releases, but is not researched or verified in any way whatsoever to ensure the publicly available information is correct. Furthermore, MEC Systems LLC often employs independent contractor writers who may make errors when researching information and preparing these communications regarding profiled companies. Independent writers’ works are double-checked and verified before publication, but it is certainly possible for errors or omissions to take place during editing of independent contractor writer’s communications regarding the profiled company(s). You should assume all information in all of our communications is incorrect until you personally verify the information, and again are encouraged to never invest based on the information contained in our written communications. The information in our disclaimers is subject to change at any time without notice. See full disclaimer at http://milestonecapitalgrowthportfolio.com/terms-conditions-of-use/.